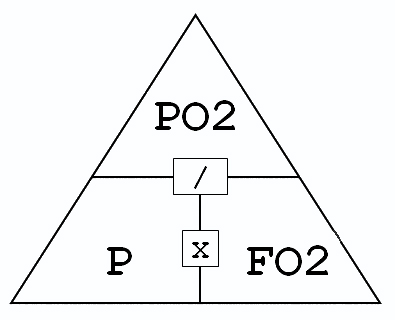
 Dalton's Triangle or more accurately Dalton's Law simply states...
Dalton's Triangle or more accurately Dalton's Law simply states..."The total pressure exerted by a mixture of gasses is equal to the sum of the pressures that would be exerted by each of the gases if it alone were present and occupied by the total volume."
How the "triangle" is used is if you know two of the values you can determine the third value, cause maths. The formula shows the relationships between partial pressure (PO2), fraction of oxygen (FO2) expressed as a decimal and ambient pressure (P)..or for us scuba divers...depth.
- To go from ATA to depth you take ATA minus 1 and multiple by 33...(4 ATA - 1) * 33 = 100ft
The conservative PO2 for recreational divers is 1.4
The maximum PO2 for recreational divers is 1.6 (hint: this matches Nitrox 32% @ 130ft)
What is the maximum operating depth (MOD) for a specific O2%? (FO2)
How about for Nitrox32
- PO2 = 1.4
- FO2 = 0.32
- PO2 / FO2 = P (depth)
- 1.4 / 0.32 = 4.375 ATA
- (4.375 - 1) * 33 = 111 ft
- MOD for Nitrox32 is ~110ft
How about for Air (21% O2)
- PO2 = 1.4
- FO2 = 0.21
- PO2 / FO2 = P (depth)
- 1.4 / 0.21 = 6.667 ATA
- (6.667 - 1) * 33 = 187 ft
- MOD for Air is ~190ft
If Recreational Depth Limit is 130ft then what is the optimal O2% for that depth?
- PO2 = 1.4
- Depth = 130 ft
- P = (130 / 33) + 1 = 5 ATA
- PO2 / P = FO2 (Max % of O2 in our Nitrox blend)
- 1.4 / 5 = 0.28 : 28%
- Optimal Nitrox blend for 130 ft is 28%
Hang on...isn't the standard banked Nitrox for recreational diving 32%?
- Depth = 130 ft
- P = (130 / 33) + 1 = 5 ATA
- FO2 = 0.32
- P x FO2 = PO2
- 5 x 0.32 = 1.6 PO2
